19+ rydberg formula calculator
Admission How to enroll. Why Why choose us.

Monash Chemistry The Rydberg Equation Youtube
The Rydberg Equation is used to describe the wavelengths of the spectral lines of many chemical elements in atomic physics.

. The Rydberg formula is a mathematical formula used to predict the wavelength of light resulting from an electron moving between energy levels of an atom. The Rydberg formula is a mathematical formula for calculating the wavelength of light emitted by an electron moving between the energy levels of an atom. The Rydbergs Equation for hydrogen is used to determine the wavelength of light emitted by an electron moving between the energy levels of an atom with an atomic number of hydrogen.
The Rydberg formula determines the wavelength of an electron state defined by the quantum number n. N1 and n2 are integers. H c λ h c λ 2176 10 19 1 n 1 2 1 n 2 2 Where h Plancks constant 66 10-34.
λ Wavelength of the emmited light electromagnetic rediation in the vacuum. C Speed of light 3 108 ms. Rydberg Equation Calculator.
It is used to calculate the wavelength of the electromagnetic. Rydberg Constant Formula. 1wavelength of the photon emitted Rydberg constant 1 integer 2 2 1.
R Rydberg Constant 1097x 107 m-1. It is a wavenumber association with the electromagnetic spectrum of each element. Wavelength of Electromagnetic Radiation Emitted in Vacuum.
Z Number of proton in the nucleus of. According to Rydbergs formula. The Rydberg Equation Calculator is a tool that helps in the calculation and comprehension of the hydrogen emission spectrum.
Use our online rydberg equation calculator tool to calculate the wavelength of the light. The Balmer-Rydberg Equation calculator computes the wavelength corresponding to the hydrogen atoms energy level differences when an electric current is passed through hydrogen. Rydberg formula predicts the wavelength of light.
Along with the successive Bohrs model Rydberg. Courses See our courses. Rydberg Formula Calculator Normal View Full Page View.
Timetable Check it out. The Rydbergs Equation is used to determine the wavelength of light emitted by an electron moving between the energy levels of an atom is calculated using Wave Number of Particle. λ vac R1n 1 2 - 1n 2 2 Where λ vac Wavelength of electromagnetic radiation emitted in vacuum R Rydberg Constantapproximately 1097 x 10 7 m-1 n 1 n 2 Integers.
Rydberg Equation Calculator Results.

Calculate Absorption Rydberg Youtube
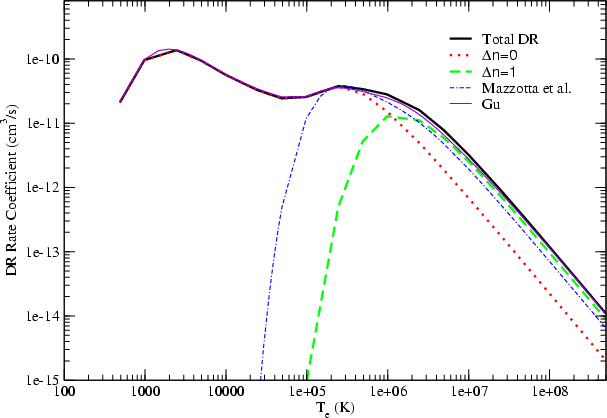
Astronomy Astrophysics
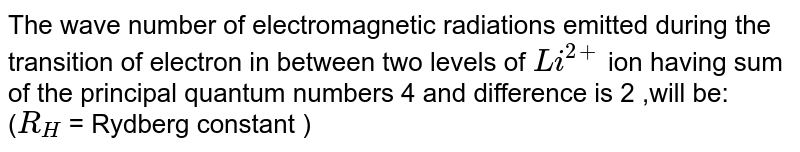
Calculate The Wavelength Emitted During The Transition Of An Electron In Between Two Level Of Li 2 Ion Whose Sum Is 4 And Difference Is 2
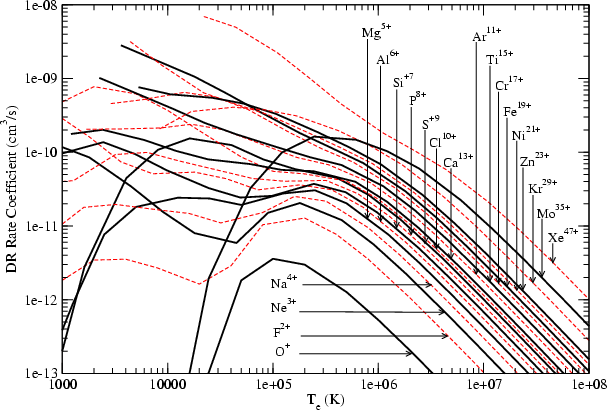
Astronomy Astrophysics

Description Of Plasmon Like Band In Silver Clusters The Importance Of The Long Range Hartree Fock Exchange In Time Dependent Density Functional Theory Simulations The Journal Of Chemical Physics Vol 141 No 14
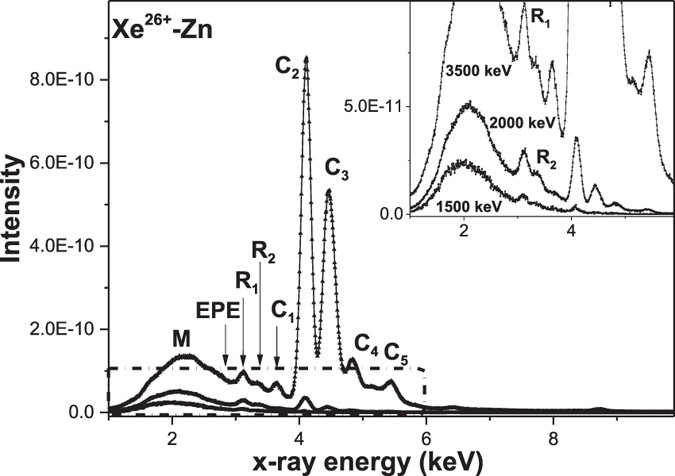
The Continuous And Discrete Molecular Orbital X Ray Bands From Xeq 12 Q 29 Zn Collisions Scientific Reports

Rydberg Equation Calculator Calculator Academy

Experimental And Theoretical Fe Xxi Target Energies Relative To The Download Table

Isoelectronic Sequence An Overview Sciencedirect Topics
Download Vol 5 As Pdf Facility For Antiproton And Ion Research
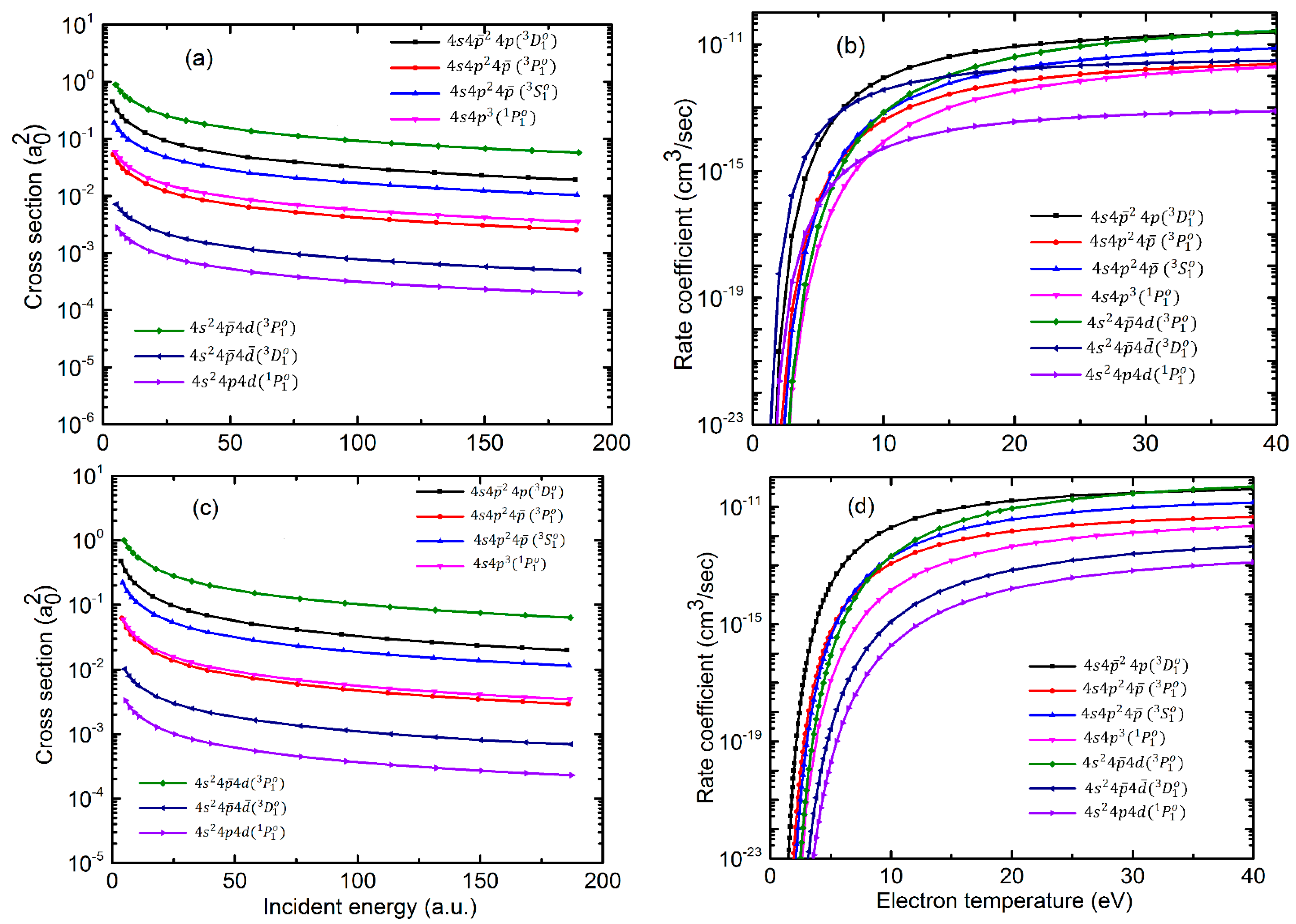
Atoms Free Full Text Electron Impact Excitation Of Ge Like Te20 Ndash Cd16 Ions Html
Suppose That An Electron Starts In The N 4 Shell Of A Neutral Hydrogen Atom How Many Photons Will Be Emitted Once It Has Fallen To The N 1 Shell Quora

Using Rydberg S Formula Youtube

Dielectronic Recombination Of The Open 4d Shell Of Tungsten W37 W28 Iopscience

Rydberg Equation Calculator Free Calculator To Find Rydberg Equation
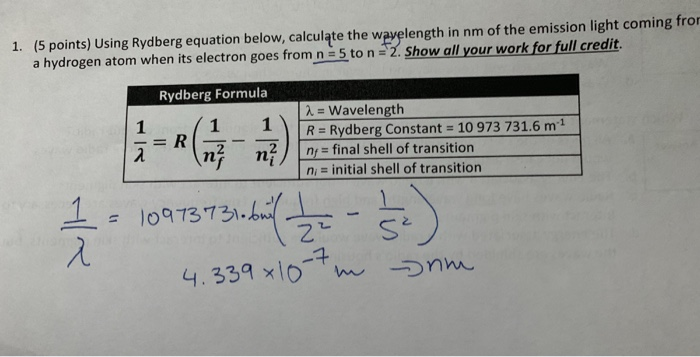
Solved 5 Points Using Rydberg Equation Below Calculate Chegg Com
Suppose That An Electron Starts In The N 4 Shell Of A Neutral Hydrogen Atom How Many Photons Will Be Emitted Once It Has Fallen To The N 1 Shell Quora